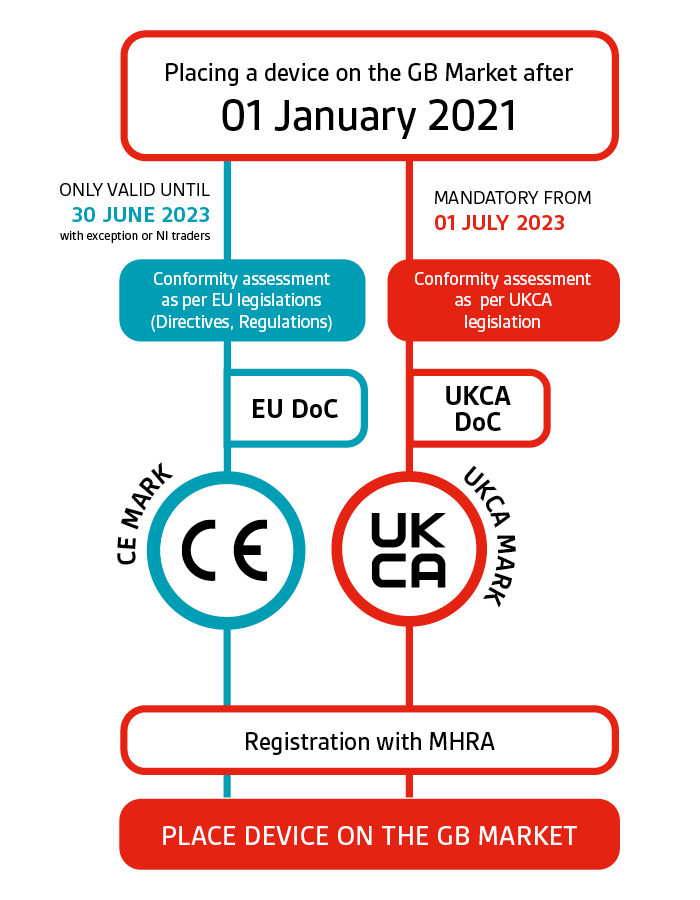
Ukca Labelling Medical Devices . find out if you need to use the ukca (uk conformity assessed) marking on products you manufacture or handle. to get a ukca mark for your medical device you should: the ukca mark can be affixed to medical devices from 1st january 2021. Work out the risk classification for your device. read our fact sheet to find out: Importantly however, it should be noted that whilst the. Work out the risk classification for your device. to get a ukca mark for your medical device you should: Read all the relevant legislation. medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. Ukca versus ce marking technical file and labeling differences; the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk.
from www.bsigroup.com
to get a ukca mark for your medical device you should: the ukca mark can be affixed to medical devices from 1st january 2021. to get a ukca mark for your medical device you should: Read all the relevant legislation. Work out the risk classification for your device. Importantly however, it should be noted that whilst the. read our fact sheet to find out: Work out the risk classification for your device. medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. Ukca versus ce marking technical file and labeling differences;
UKCA marking for medical devices certification BSI
Ukca Labelling Medical Devices Work out the risk classification for your device. to get a ukca mark for your medical device you should: Importantly however, it should be noted that whilst the. the ukca mark can be affixed to medical devices from 1st january 2021. medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. Work out the risk classification for your device. Work out the risk classification for your device. to get a ukca mark for your medical device you should: find out if you need to use the ukca (uk conformity assessed) marking on products you manufacture or handle. Ukca versus ce marking technical file and labeling differences; read our fact sheet to find out: the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk. Read all the relevant legislation.
From www.productapprovals.co.uk
UKCA marking UK Ukca Labelling Medical Devices find out if you need to use the ukca (uk conformity assessed) marking on products you manufacture or handle. to get a ukca mark for your medical device you should: Importantly however, it should be noted that whilst the. Work out the risk classification for your device. medical devices placed on the great britain market must have. Ukca Labelling Medical Devices.
From www.castoredc.com
UKCA Mark Impact of Brexit on Medical Devices Castor Ukca Labelling Medical Devices Importantly however, it should be noted that whilst the. Work out the risk classification for your device. find out if you need to use the ukca (uk conformity assessed) marking on products you manufacture or handle. Work out the risk classification for your device. medical devices placed on the great britain market must have a ukca marking or. Ukca Labelling Medical Devices.
From medicaldevices.freyrsolutions.com
UKCA Marking Requirements for Medical Devices Freyr Medical Devices Ukca Labelling Medical Devices read our fact sheet to find out: Work out the risk classification for your device. the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk. to get a ukca mark for your medical device you should: Work out the risk classification for your device. to get a ukca mark. Ukca Labelling Medical Devices.
From www.thelabelpeople.co.uk
UKCA Labels with Company Logo 50mm x 25mm The Label People Ukca Labelling Medical Devices Importantly however, it should be noted that whilst the. Ukca versus ce marking technical file and labeling differences; Read all the relevant legislation. to get a ukca mark for your medical device you should: to get a ukca mark for your medical device you should: Work out the risk classification for your device. find out if you. Ukca Labelling Medical Devices.
From fra.animalia-life.club
Iso 15223 1 Symboles Ukca Labelling Medical Devices Work out the risk classification for your device. find out if you need to use the ukca (uk conformity assessed) marking on products you manufacture or handle. medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. the ukca mark can be affixed to medical devices from 1st. Ukca Labelling Medical Devices.
From touche-tech.com
Product labelling for UKCA and CE Touché Technology Ukca Labelling Medical Devices to get a ukca mark for your medical device you should: to get a ukca mark for your medical device you should: the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk. read our fact sheet to find out: Read all the relevant legislation. Work out the risk classification. Ukca Labelling Medical Devices.
From www.fobalaser.com
UKCA marking on medical devices New regulations Ukca Labelling Medical Devices the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk. read our fact sheet to find out: to get a ukca mark for your medical device you should: Read all the relevant legislation. find out if you need to use the ukca (uk conformity assessed) marking on products you. Ukca Labelling Medical Devices.
From www.tuvsud.com
UKCA Marking for Medical Devices TÜV SÜD Ukca Labelling Medical Devices the ukca mark can be affixed to medical devices from 1st january 2021. Work out the risk classification for your device. to get a ukca mark for your medical device you should: Importantly however, it should be noted that whilst the. medical devices placed on the great britain market must have a ukca marking or a ce. Ukca Labelling Medical Devices.
From www.i3cglobal.com
UKCA Marking Consultants For Medical Devices & IVDs Ukca Labelling Medical Devices Work out the risk classification for your device. find out if you need to use the ukca (uk conformity assessed) marking on products you manufacture or handle. Work out the risk classification for your device. to get a ukca mark for your medical device you should: read our fact sheet to find out: Read all the relevant. Ukca Labelling Medical Devices.
From www.bsigroup.com
UKCA marking for medical devices certification BSI Ukca Labelling Medical Devices Ukca versus ce marking technical file and labeling differences; Work out the risk classification for your device. to get a ukca mark for your medical device you should: the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk. Read all the relevant legislation. Work out the risk classification for your device.. Ukca Labelling Medical Devices.
From www.cognidox.com
What to know about UKCA Marking for Medical Devices Ukca Labelling Medical Devices Ukca versus ce marking technical file and labeling differences; to get a ukca mark for your medical device you should: read our fact sheet to find out: the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk. Read all the relevant legislation. find out if you need to use. Ukca Labelling Medical Devices.
From www.youtube.com
UKCA Marking for medical Devices YouTube Ukca Labelling Medical Devices read our fact sheet to find out: the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk. Work out the risk classification for your device. to get a ukca mark for your medical device you should: Work out the risk classification for your device. to get a ukca mark. Ukca Labelling Medical Devices.
From shirobusiness.com
UKCA Marking for Northern Ireland Shiro Business Solutions Ukca Labelling Medical Devices Ukca versus ce marking technical file and labeling differences; to get a ukca mark for your medical device you should: medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk. . Ukca Labelling Medical Devices.
From www.productcompliancesupport.co.uk
Templates for UKCA and CE Marking Product Compliance Support Ukca Labelling Medical Devices find out if you need to use the ukca (uk conformity assessed) marking on products you manufacture or handle. Work out the risk classification for your device. Ukca versus ce marking technical file and labeling differences; Importantly however, it should be noted that whilst the. to get a ukca mark for your medical device you should: Read all. Ukca Labelling Medical Devices.
From www.bsigroup.com
Medical Devices brochures BSI America Ukca Labelling Medical Devices find out if you need to use the ukca (uk conformity assessed) marking on products you manufacture or handle. to get a ukca mark for your medical device you should: the ukca mark can be affixed to medical devices from 1st january 2021. Read all the relevant legislation. Importantly however, it should be noted that whilst the.. Ukca Labelling Medical Devices.
From www.exoltech.net
Know more about UKCA marking for Medical Devices Ukca Labelling Medical Devices read our fact sheet to find out: the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk. medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. the ukca mark can be affixed to medical devices from 1st january 2021.. Ukca Labelling Medical Devices.
From edu.svet.gob.gt
Understanding The Differences Between CE Marking And UKCA Ukca Labelling Medical Devices to get a ukca mark for your medical device you should: the ukca mark can be affixed to medical devices from 1st january 2021. the ukca marking is a certification mark that indicates a product, including medical devices, conforms to uk. to get a ukca mark for your medical device you should: Work out the risk. Ukca Labelling Medical Devices.
From operonstrategist.com
UKCA Compliance A StepbyStep Guide for Medical Device Manufacturers Ukca Labelling Medical Devices find out if you need to use the ukca (uk conformity assessed) marking on products you manufacture or handle. medical devices placed on the great britain market must have a ukca marking or a ce marking, depending on. Read all the relevant legislation. Ukca versus ce marking technical file and labeling differences; Work out the risk classification for. Ukca Labelling Medical Devices.